Pure Water Annie’s FAQ Series.
Pure Water Gazette Technical Wizard Pure Water Annie Answers All the Persistent Questions about Water Treatment.![pwanniemedium[1]](http://www.purewatergazette.net/blog/wp-content/uploads/2012/05/pwanniemedium1-246x300.jpg)
Aer-Max aeration systems, for treatment of iron and hydrogen sulfide.
How does Aer-Max work?
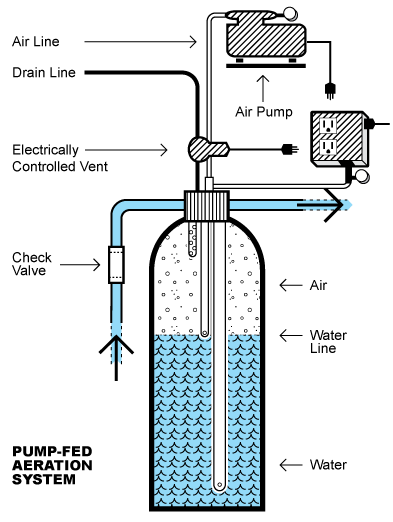
Aer-Max works by providing a pocket of compressed air in the top third of a closed tank. When water containing hard-to-treat contaminants like iron, manganese, and hydrogen sulfide falls through the air pocket, the contaminants are oxidized so they can be easily removed by a filter. The compressed air is supplied by a small air pump. A vent is provided to keep the air pocket fresh. The Aer-Max system is not a filter. It prepares contaminants for easy removal by a filter that follows the aeration tank.
There are 110-volt and 220-volt systems. Which is better?
The voltage needed depends mainly on how the unit is to be controlled. The pump and vent can be turned off and on by having them wired directly into the electrical circuit that turns the well pump off and on. Since most well pumps run on 220 volt current, if you choose this method of control it’s easiest to use a 220-volt Aer-Max. If you use an alternative control system, like a flow switch or a simple timer, however, you would want the 110-volt system.
Which is the best way to control the system?
For residential use, the flow switch is the last choice. It turns the Aer-Max unit on when water flows through the pipe toward the home. Usually this results in frequent and short on/off cycles and is the least efficient way to operate the system. The conventional method is to bring 220-volt electrical receptacles for the air pump and the vent solenoid out of the pressure switch that controls the well pump. With this system, the AerMax is activated when the well pump runs and turns off when the well pump is not running. This is a proven system and it works well. Use of a simple timer, the kind used to turn lamps off and on at specified times, is becoming the most popular, however. It’s easiest to install: you just plug the pump and vent solenoid into the timer and plug the timer into a wall receptacle.
Standard Aer-Max System
But doesn’t the air pump have to be running while water is going through the treatment tank?
This is probably the biggest source of misunderstanding about how Aer-Max works. The rich pocket of compressed air in the top of the treatment tank needs only to be refreshed from time to time: effective treatment does not depend on fresh air entering while water is running through the tank. With hydrogen sulfide, for example, while a small amount of the offensive gas may be vented out of the tank by the drain system, treatment consists mainly of reducing the odor-causing gas to elemental sulfur so that it can be removed by the filter that follows the air treatment tank. Residential users who control the unit with timers usually run the air pump only about three times a week. This vents the tank and refreshes the air pocket. Unless you run large amounts of water, three times a week is enough.
What is the function of the three tubes attached to the aeration head in the illustration above?
From left, the first, the shortest, is a baffling device. It creates turbulence to enhance aeration as water entering the tank falls through the air pocket. The middle tube is the vent tube. It maintains the level of the air pocket in the tank. The long tube is the pickup tube for treated water being sent to the home.
What kind of filter has to be used after the Aer-Max?
Aer-Max enhances the performance of any standard iron filter medium. It works especially well with Birm, Filox, and Katalox Light. Media like Zeolite (Turbidex) and Filter Ag can be used as iron filters if the water is pre-treated with Aer-Max, and an especially effective treatment for both iron and low pH can be accomplished by using a backwashing calcite filter after Aer-Max. For large amounts of iron it’s best to use the best–Filox or Katalox Light. Both media will treat both iron and hydrogen sulfide after aeration.
Both Filox and Katalox Light work well with both hydrogen sulfide and iron. If hydrogen sulfide is present, Birm is not a good choice.
For hydrogen sulfide, catalytic carbon is the best available, but standard carbon also works well. Actually, any granular filter medium will remove odor after AerMax, but carbon is best. If no iron or manganese is present, a cartridge style carbon filter (4.5″ X 20″ preferred) can be used to treat hydrogen sulfide odor, but a cartridge filter will stop up quickly if there’s iron in the water. With iron and manganese, a backwashing filter is required.
Standard Air Pump Used for Aer-Max
I’m using a water softener to remove iron, but it isn’t quite doing the job. Can I install an Aer-Max unit in front of it to improve its performance?
No. The Aer-Max will actually interfere with the softener’s ability to remove iron by turning the ferrous iron to ferric. Filters catch ferric iron easily, but a softener is an ion exchanger, not a filter. If your water is hard and has iron, either remove both with the softener or use both filter and softener. The correct order of treatment if aeration is used is Aer-Max, iron filter, then softener.
How loud is the pump?
Approximately 50 decibels.
How long does the pump last, and does it need regular maintenance?
The pump usually runs 20,000 to 25,000 hours before bearings need replacement. It’s an easy pump to work on, and parts are available. The most common maintenance issue is cleaning. Although the pump has an air filter, in some environments it will need an occasional cleaning (see instructions).
Which works best — Aer-Max or the newer style single tank aeration/filtration systems that are becoming popular?
Aer-Max and the newer single tank units, which have the filter and the aeration treatment in the same tank, work on exactly the same principle but there are some significant differences. In general, the Aer-Max is more robust and will handle higher contaminant levels and higher flow rates. It can also be used to pre-treat for multiple filters. Single-tank setups, which use a venturi draw rather than an air pump, are more compact (one tank rather than two), easier to install, and less expensive to purchase. Once installed, both systems are low maintenance unless high levels of iron are involved. Any equipment removing iron will eventually need some cleaning. The Aer-Max plus filter arrangement is definitely preferred over single tank units for large amounts of iron or hydrogen sulfide–over 8 parts per million of either.
Does the Aer-Max have to be vented outdoors when treating hydrogen sulfide because of the odor?
Odor isn’t an issue, but water is. The 3/8″ drain tube will vent both air and water when when the vent valve is open, so it needs to be connected to a suitable drain. It is often teed into the drain tube or pipe that serves the backwashing filter, but it can simply be allowed to drain onto a lawn or water a shrub. The drain water isn’t toxic.
Is there only one size Aer-Max system?
No, there is a high capacity pump available and it can be used with larger aeration tanks to create high flow systems. The higher capacity pump is usually preferred on “constant pressure” wells, where a higher air pressure needs to be maintained in the treatment tank. The 10″ X 54″ treatment tank, with the standard air pump, works in almost all residential applications, so it the the system most frequently sold. See high capacity systems here.