Removing Nitrates at Point of Entry with Anion Exchange Resin
As nitrate contamination of US wells, including those used for city water supplies, becomes more common, the need for nitrate treatment is becoming more urgent. The acceptable limit for nitrates is 10 mg/L and this limit is being exceeded with greater frequency because of excessive fertilizer use and contamination from animal farms and feedlots.
Removing nitrates from drinking water is fairly simple, with reverse osmosis being a proven performer with nitrates. Distillation is also very effective with nitrates.
For point of entry applications, however, the price of equipment and operation goes up sharply, with the main strategy being anion exchange. An anion exchanger works a lot like a water softener, but it costs more to purchase and operate. In addition, there are some pitfalls to avoid.
Here’s some advice from a Penn State University publication:
Once a water supply becomes contaminated with nitrate, it is costly to treat. While treatment to meet drinking water needs is practical, treatment of larger quantities like livestock supplies is costly. Ion exchange units, reverse osmosis, or distillation all remove nitrate from drinking water. Note that boiling water does not remove nitrates and is not a treatment alternative. In fact, it increases nitrate concentrations as water evaporates.
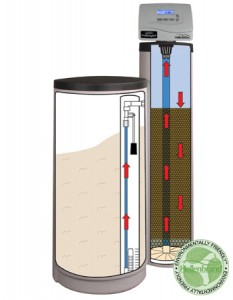
An ion exchange unit operates much like a household water softener. A softener filters calcium and magnesium laden water through a resin coated with sodium ions. As water flows through the unit, the resin releases its sodium ions and readily trades them for the calcium and magnesium. For nitrate removal, the resin exchanges chloride ions for nitrate and sulfate ions in the water. After treating many gallons of water, the resin will “run out” of chloride. Regenerating the resin with a concentrated solution of sodium chloride (you can use bicarbonate instead of chloride) recharges it for further treatment.
Ion exchange does have drawbacks. Because the resin prefers to absorb sulfate, water high in sulfates hinders the nitrate exchange and reduces system effectiveness. If the resin becomes saturated, it releases the nitrates in place of sulfates, resulting in an increased nitrate concentration in the “treated” water. Also, nitrate ion exchange can make the water corrosive. Neutralizing the water after it leaves the unit reduces this effect. Finally, ion exchange can be expensive and requires maintenance. Since the backwash brine will be high in nitrates, care must be given to its disposal.
Source: Penn State Extension.